The class 1 new durg X842 that used to treat gastroesophageal reflux disease
The SND001 class 1 new drug X842 potassium competitive acid blocker(P-CABs) is a new structure, new mechanism, best in class new antacid drug with a market size of $25 billion. It is used for the treatment of refractory reflux esophagitis that cannot be met by proton pump inhibitors(PPI).
This project was included in the National Major Scientific and Technological Special Project for “Significant New Drugs Development” in July 2014 (2015GKH-204) and approved by the EU phase I clinical in January 2017, then obtained the China food and drug administration Clinical Trial Permission(CTP) in April 2008. Currently, the phase II clinical research on erosive esophagitis have been carried out in 15 domestic clinical trial institutions.
Sentinel Lymph Node T cell(SLN-T) vaccine
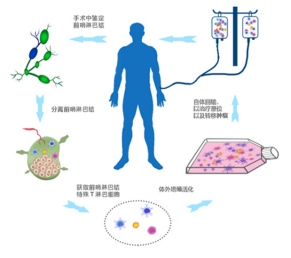
The SND002 Sentinel Lymph Node T cell(SLN-T) vaccine has been approved to start the EU phase II clinical trial, and was included in the National Major Scientific and Technological Special Project for “Significant New Drugs Development” in 2013. SLN-T is a individualized cell therapy drug with tumor specificity, the result of preliminary multicntre exploratury clinical trials proved that it can significantly prolong the survival of patients with stage IV colorectal cancer. The Karolinska medical school in Sweden has been doing relevant research for around 10 years, with an investment of nearly 200 million Swedish kronor.
Sentinel lymph node (SLN) is the first stop of lymph node metastasis of primary tumor. Because of its special location, T cells (sln-t) are induced by tumor antigen, thus can specifically recognize tumor cells and have special anti-tumor activity. After being induced, acclimated and amplified by autologous tumor antigen(neoantigen) and cell activation factor in vitro, when put it back into the patient's body, it can play a specificity anti-tumor function and can be used to treat stage Ⅱ or above malignant solid tumors.
Oncolytic virus
SND005 oncolytic virus is a virus that preferently infects and kills tumor cells. Initially, some tumor cells were specifically infected and destroyed by oncolytic virus, and subsequently, oncolytic viruses will replicate and proliferate inside the tumor cells, then release new infectious viral particles to infect and destroy other tumor cells. The oncolytic virus performs its oncolytic function by dissolvinchan tumor cells directly or by stimulating the host to produce the anti-tumor immunologic reaction.
SND005 has a history of 14 years of clinical use, and is the only wild-type virus that has no genetic modification among current oncolytic viruses. More than 44 percent of the 540 melanoma patients in that trial benefited, and 190 patients have participated in safety and tolerability research with no serious adverse events, the most common adverse reaction was the slight fever. According to the clinical data and observations after oversea marketing, the survival rate of patients used SND005 was improved by 4-5 times, especially in stage II melanoma patients.